Abstract
Introduction. 20-25% of patients (pts) with chronic myeloid leukemia (CML) in chronic phase (CP) failure second line therapy with 2nd generation tyrosine kinase inhibitors (TKI). Many of them are non eligible for allogeneic stem cell transplantation(alloSCT) and are candidates for 3rd line TKIs (TKI3). Thus,it is important to evaluate predictors of 3rd line TKI efficacy.
Methods: Prognostic factors for TKI3 were evaluated in 43 pts (male -17; 39,5%) with CML in CP. Median age was 51 (range 23-88)years. All pts obtained Imatinib as first line treatment for a median 17 (range 1-85)months. Second line TKI were Nilotinib in 25 (58%), Dasatinib in 14 (33%) and Bosutinib in 4 (9%) of pts. Median duration of second TKI therapy was 13 (range 1-96) months. The main reason for treatment discontinuation was resistance for both first (35;81%) and second (38; 88%) TKIs.Mutations were revealed before second or third line TKI in 6/21(29%) and 12/28 (43%) pts, respectively, including 3pts with T315I mutation. Additional chromosomal abnormalities (ACAs) were observed in 9/36 pts. At baseline before TKI3 the best cytogenetic response was complete (CCyR), partial (PCyR), minimal or minor (mCyR), no CyR in 7(16%) 6(14%), 8(19%) pts and 22(51%), respectively. Complete hematologic response (CHR) without any CyR was observed in 13/22 (30%) pts.
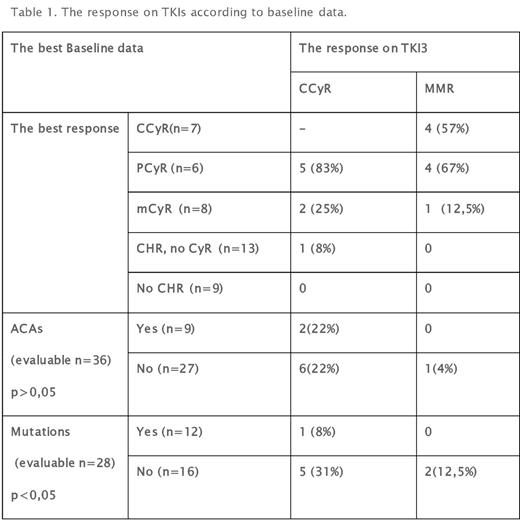
Results: Nilotinib, Dasatinib or Bosutinib were used as TKI3 in 16(37%), 22(51%) and 5(12%) cases, respectively. Median time from diagnosis to TKI3 was 56 (range 13-314) months. Median observation time from TKI3 to data of analysis is 22 (range 8-119) months. Overall CCyR was obtained 8/36 pts (22%) pts without CCyR at baseline. CCyR was reached in 7/14(50%) and only 1/22 (4,5%) pts with any level CyR and no CyR, respectively (p=0,003). CHR obtained 7/9 (78%), but 3/7 (43%) subsequently lost the response within a median of 6 months follow-up. The cumulative rate of CCyR and major molecular response (MMR) on TKI3 according to baseline data are presented in table1. Nearly all patients (5/6; 83%) with PCyR before TKI3 responded. Progression to accelerated (AP) or blastic (BP) phase was observed in 10/22(45%) and 0/21 pts with no CyR or any level CyR at baseline(p=0,0003). Other unfavorable factorsfor disease progression were mutations. AP/BP were observed in 7/12 (58%) and 2/16 (12,5%)(p=0,015)with and without mutations, respectively. Any ACAs 4/9(44%) and 6/27(22%)(p=0,19) More than half of patients 25/43(58%) discontinued TKI3 - median treatment time was 16 (range 2-55)months. Reasons for treatment withdrawal were resistance (16; 64%), toxicity (4;16%), alloSCT (5;20%).
Conclusions: According to our study pts with any cytogenetic response, especially with PCyR, and without mutations at baseline benefit 2nd generation TKIs as third line treatment. Thus, in case of alloSCT noneligibility, this cohort of pts are good candidates for 2nd generation TKI as 3rd line treatment.
Shuvaev: Fusion Pharma LLC: Consultancy. Zaritskey: Novartis: Consultancy, Speakers Bureau; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal